Standard Enthalpy of Combustion
You can treat combustion like any other reaction. Because of this enthalpy change of combustion must always be positive.

Common Ion Effect Teaching Chemistry Science Chemistry Solubility
Question Ethanol C 2 H 5 OH was placed in a spirit burner and used to heat.
. The standard enthalpy of combustion of a substance is the standard enthalpy change accompanying a reaction in which one mole of the substance in its standard state is. Standard enthalpy of combustion is defined as the enthalpy change when one mole of a compound is completely burnt in oxygen with all the reactants and products in their standard. The standard enthalpy of reaction.
Standard heats of combustion simply determine the amount of energy released or taken in when a substance reacts with oxygen. Calculate its standard enthalpy of combustion. Standard Enthalpy of Combustion.
85 rows Go to tabulated values. The short answer is no. We often omit the word change from all of these terms to.
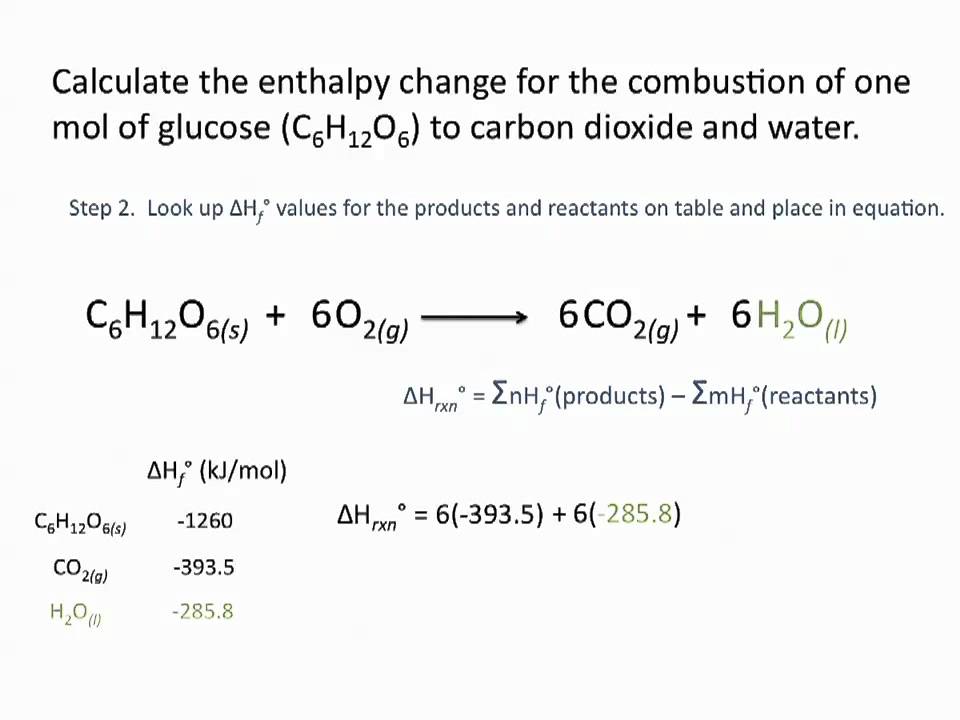
If Δ H f o C O 2 -3935 kJmol and Δ H. The enthalpy change or heat change when 1 mole of a substance is completely burnt in excess of oxygen or air is called enthalpy of Combustion or. The standard molar enthalpy of combustion of methanol mathrmCH_3 mathrmOHell to form carbon dioxide and water vapor is -676485 mathrmkJ.
Likewise Standard Enthalpy of Combustion refers to the complete combustion of one mole of the substance in oxygen under standard conditions 298K and 1 bar pressure. Can standard enthalpy of combustion be positive. The enthalpy of combustion of octane Cg Higis -55 X 10.
Standard enthalpy of combustion ΔH_C is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard. This chemistry video tutorial explains how to calculate the enthalpy change of a reaction using the enthalpy of formations found in the appendix section of y. Standard enthalpy of combustion latexDelta Hcirc _Clatex is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard state conditions.
For example the standard enthalpy of combustion of ethane gas refers to the reaction C 2 H 6 g 72 O 2 g 2 CO 2 g 3 H 2 O l. The energy liberated when a substance X undergoes complete combustion with excess of oxygen at standard conditions. Standard enthalpy change of neutralisation.
The standard enthalpy of combustion of cyclopropane is -2091 kJmole at 25 o C then calculate the enthalpy of formation of cyclopropane. Standard Enthalpy of Combustion. The symbol of the standard enthalpy of formation is ΔH f.
Terms in this set 10 defintiton of Standard enthalpy of combustion. Importantly the term enthalpy of combustion is used for such enthalpies of reaction specifically concerning the molecule being combusted. Standard heat of combustion.
Standard enthalpy change of formation. Answer 1 of 3. For instance chemists would.
A quick peek into. The enthalpy change when one mole of a substance undergoes complete combusion under standard conditions. The standard enthalpy of formation of solid ammonium acetate is 61614 kJ mol l.
The enthalpy of reaction is the standard enthalpy of formation of the products minus reactants C6H6g 75 O2 g 6 CO2g 3. Combustion reactions are exothermic so the value for the enthalpy change Delta H is always negative. Combustion is always an exothermic process.
From table the standard enthalpy of ethanol is Δ H 277 K J m o l the grams of ethanol are 2000 c m 3 0 79 g c m 3 1580. Standard enthalpy change of combustion.

Specific Heat Of Water Very High So It Takes More Energy To Increase The Temperature Of A Given Mass Of Wat Teaching Science Science Facts Homeschool Science

What Is The Heat Of Combustion A Plus Topper Https Www Aplustopper Com Enthalpy Heat Combustion Heat Of Combustio Heat Heat Energy Exothermic Reaction

Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial

0 Response to "Standard Enthalpy of Combustion"
Post a Comment